The risk to the mother and the unborn child from maternal exposure during pregnancy is part of every risk management plan for a newly approved small molecule. Here we look at the causality assessment of birth defects.
Birth defects are generally classified as major or minor defects. A major defect is an abnormality of structure, function or metabolism that is either fatal or present at birth and results in physical or mental disability. The term minor defect generally refers to minor physical anomalies that represent deviations from what is considered normal and do not have obvious medical, surgical or cosmetic consequences.
Birth defects are also classified into four mechanistic types. Malformations are a single, localized, poor formation of tissue that initiates a chain of subsequent defects. This, typically, results from a chromosomal abnormality or gene mutation during gametogenesis. Deformations occur when a normal embryo/foetus is subject to mechanical forces such as uterine constraints resulting in a birth defect. Disruptions occur when a normal embryo/foetus is subject to disruptive forces that may be vascular, infections or of mechanical origin. Dysplasia is the lack of normal organization of cells – typically seen in the nervous system.
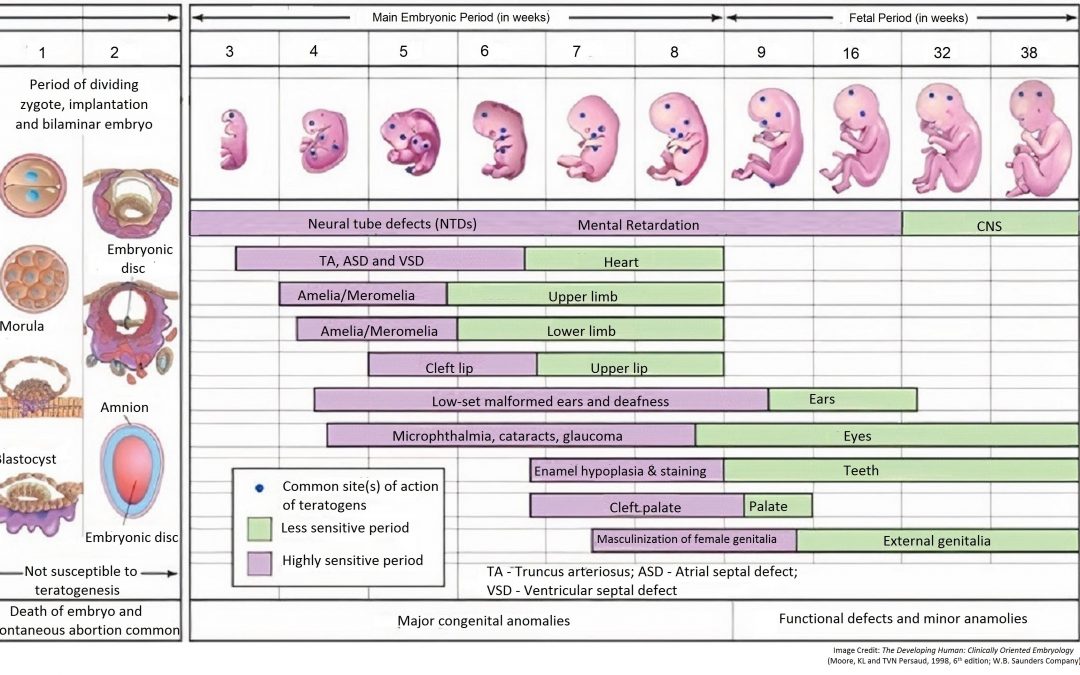
In a single case analysis, it is important to know the time of exposure to the drug for assigning causality to a birth defect. Exposure to the drug may occur during gametogenesis (before conception), pre-implantation period (first two weeks), embryonic period (weeks three to eight) or the foetal period (week nine through birth).
A birth defect may result during gametogenesis if the drug is a mutagen. During gametogenesis, two chromosomes of a pair, instead of separating, may both go to the same gamete. This may result in a zygote with a trisomy (47 chromosomes) or a monosomy (45 chromosomes). Abnormal gametogenesis may also result in a translocation (part of a chromosome may get attached to a chromosome of a different pair) or a deletion (part of a chromosome is lost). In most cases, these aberrations result in a spontaneous abortion in the first trimester, though, occasionally, the embryo may survive to term (trisomy 21 – Down syndrome; 45 XO Turner syndrome). In some instances, the abnormality during gametogenesis may be restricted to a gene mutation. These could be ‘spontaneous’ during gametogenesis or inherited and unrelated to gametogenesis. The foetal outcome depends on the mutation (dominant or recessive) and the sex of the foetus (autosome, sex chromosome). Irrespective of whether the abnormality is inherited or results during gametogenesis, it is seen in all cell lines of the body. A birth defect associated with an inherited genetic defect is not related to drug. All other birth defects associated with a genetic defect may be drug related if there was exposure to the drug prior to conception and if the drug is known to be a mutagen.
In the pre-implantation period (first two weeks), the developing embryo is not susceptible to teratogenesis. Drug exposures during this time are not known to cause birth defects, though such exposures may cause early death and spontaneous abortion of the embryo.
In the embryonic period (weeks 3 to 8), the embryo is most easily disrupted. Disruption may occur with drug, diabetes (e.g. sacral agenesis) and infections (e.g. cytomegalovirus, rubella). Disruption during this period may induce gross structural abnormalities readily seen at birth. It is critical to understand the nature and timing of disruption. The development of the heart occurs between week 3 and 6. In the analysis of a major cardiac birth defect, if exposure to the drug occurred after week 6, the drug cannot have been the cause of the birth defect.
In the foetal period (week 9 to term) exposure (to drugs and disease) may cause intrauterine growth retardation (IUGR) or functional disorders such as mental retardation (dysplasia) or minor birth defects.
Using this approach, we were able to review birth defects for an immunosuppressant and identify ear malformations as being caused by exposure to the drug, with a change in the label.
For more blogs, please visit: https://www.rxmd.com/insights