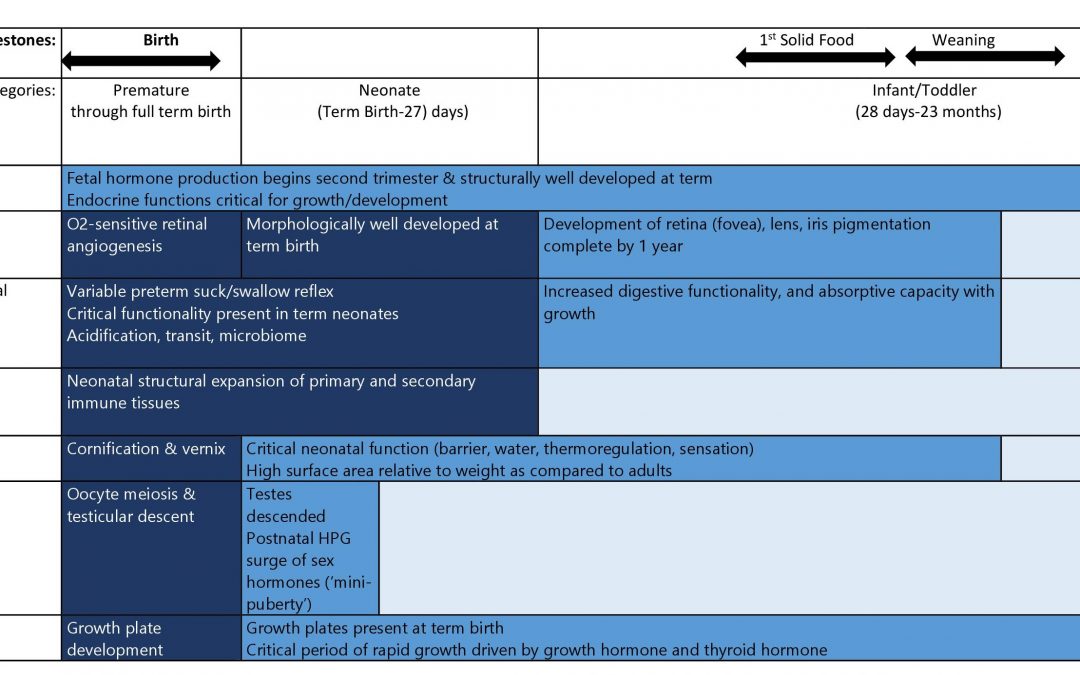
This blog is a continuation of a previous blog on the safety issues that might be uncovered in the pediatric population. Here we look at susceptibilities particular to the first two years of life for some key organ systems. The table above depicts the key developmental changes for other organ systems.
Cardiovascular System: New born heart is characterized by near-maximal basal ß-stimulation and lower myocardial compliance. This results in lower functional cardiac reserve. An increase in cardiac output is achieved by increasing heart rate initially; later, by increasing the stroke volume and systemic venous return. Children in this age group are susceptible to cardiac failure manifested by tachypnea, tachycardia, delayed capillary refill, mottled skin. These adverse events (AEs) may need to be monitored for.
Central Nervous System (CNS): CNS immaturity in this age group exists, in part, due to immaturity in the blood brain barrier (BBB), and efflux transporters. A more permeable BBB with decrease in P-glycoprotein (PGP) expression (an efflux transporter) in the brain, can result in higher concentrations of drugs in the brain than in older children and adults. The CNS AEs to watch for in this population relate to grey matter excitation: seizures, hallucinations; grey matter inhibition: somnolence, depressed sensorium; disturbances in white matter: delayed motor milestones, gait and balance disturbances, etc.
Increased intracerebral bilirubin concentrations, as seen in neonatal jaundice presenting with rapid rise above physiologically accepted limits, may lead to bilirubin encephalopathy and subsequently to severe brain damage (kernicterus). An interaction between drug and unconjugated bilirubin levels, reduced albumin binding capacity, and an immature BBB contribute to neuronal susceptibility. Hence drugs impacting any or all these factors can pose a potential risk of the neonate developing bilirubin encephalopathy.
Renal System: After term birth, no new nephrons are formed. Growth of the kidney to adult size is solely based on hypertrophy. A seemingly normal looking infant can harbour congenital anomalies like renal dysplasia and hypoplasia with an increased susceptibility to nephrotoxic drugs. The neonatal kidney is particularly susceptible to small changes in glomerular hydrostatic pressure which is regulated by the renin angiotensin system and circulating prostaglandins. The AEs to watch for are an increase in serum creatinine, oliguria, and fluid overload and electrolyte abnormalities.
Respiratory System: When compared to adults, infants have poor pulmonary compliance and narrower airways. Adenoids and tonsillar hypertrophy pose an additional burden to air flow. The work of breathing is increased, compensated for by a higher respiratory rate and a consequent decrease in respiratory reserve. Even short respiratory pauses (apnea) not clinically significant in adults can result in hypoxia and bradycardia. Unlike adults in whom a cardiac event is the most common terminal cause of death, in infants and children respiratory failure and arrest is the most common terminal event. Children in this age group need to be monitored for stridor, wheezing, apneas, increased work of breathing (subcostal, suprasternal retractions, tachypnea)
Liver: Birth results in changes in hepatic circulation and hepatic oxygen tension, which can affect hepatic function during the immediate post-partum period. Biliary excretory function and carrier-mediated hepatocellular uptake are sub-optimal in infants. Reyes syndrome is a rare disorder (found in older children and sometimes in this population) following a viral infection presenting with symptoms of vomiting, lethargy, encephalopathy and raised transaminases. Though the etiology is unknown, aspirin given to children has been implicated.
An understanding of the susceptibilities in this population allows for appropriate risk mitigation during pediatric drug development.
For an overview on pediatric drug development, please read our blog Pediatric Drug Development: Four Critical Considerations
References:
- https://www.ema.europa.eu/en/news/emea-workshop-neonates-development-medicines-neonates-needs-multi-disciplinary-cooperation
- ICH guideline S11 on nonclinical safety testing in support of development of paediatric pharmaceuticals
For more blogs, please visit: https://www.rxmd.com/insights