What We Do
Our skills span translational medicine, clinical development and drug safety – for small molecules, biologics and cell therapy. Our expertise encompasses strategy and execution.

Translational Medicine
Our physicians identify and bridge the gaps from in vitro pharmacology to commercialization.
Clinical Development
At every step, in every stage of clinical drug development – be it formulating the clinical development plan or first in Human, Phase 2 or pivotal Phase 3 trials – we have a skill that can be unflinchingly relied upon.
Drug Safety
We provide a wide spectrum of safety science (not operations) services in both pre-approval and post-approval stages to ensure there is no compromise on signal detection.
Client Speak

Usman "Oz" Azam, MD
In the cell and gene therapies world, RxMD have developed a very credible scientific, translational and development understanding. At Novartis during 2013-2016, my teams

Joseph H. Hoffman, MD
It is a pleasure to commend RxMD to any pharmaceutical company, large or small, that is in need of additional medical support in pursuit of its clinical research objectives.
Insights
Risk Assessment
Risk assessment is a continuous process beginning right from discovery through the life of the drug. While we collaborate closely with our discovery, non-clinical and clinical pharmacology colleagues in understanding...
Thinking Differently
Precedent often trumps science when it comes to drug development. A common refrain is, “This is what we did at xxx”, "This is how they did it” and so on. So much so that we are doing things because that was how it was...
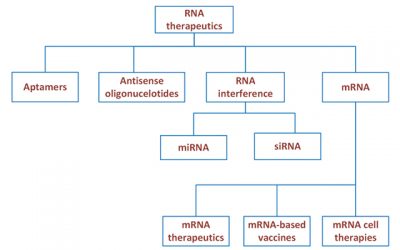
RNA Therapeutics for Monogenic Rare Diseases
There is nothing worse than handing out a diagnosis for a debilitating rare disease which has no treatment. At RxMD we find it gratifying to work on rare diseases and have worked/are working on Huntington disease, Gorlin...
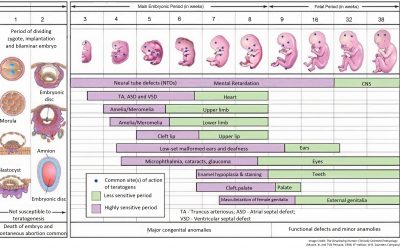
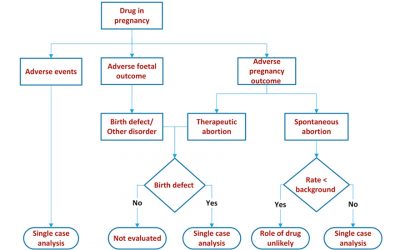
Causality Evaluation of Birth Defects
The risk to the mother and the unborn child from maternal exposure during pregnancy is part of every risk management plan for a newly approved small molecule. Here we look at the causality assessment of birth...
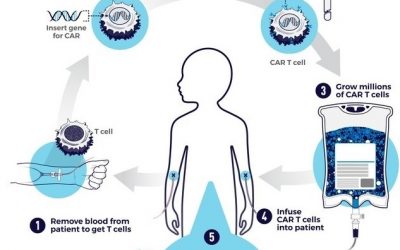
Asset Prioritization – Cell Therapy
What should one look for when prioritizing cell therapy assets? The phenomenal success of CARs in hematological malignancies has been driving the push towards development of CARs against solid tumors. We are often asked to...
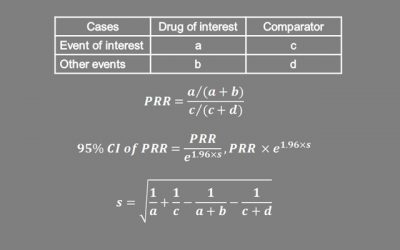
Proportional Reporting Ratio
In post-marketing surveillance, proportional reporting ratio (PRR) is a useful first step for signal detection. PRR is the ratio of the proportion of adverse events of interest with the drug versus other drugs. The...
Post-marketing Evaluation of Pregnancy Exposure
A few years ago we were requested by a Sponsor to undertake an analysis of outcomes of maternal exposures to a small molecule – a popular immunosuppressant. Of the different safety reports we author, these are particularly...
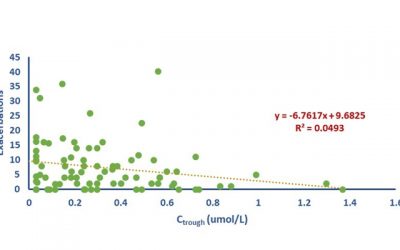
Exposure Response Analysis
An exposure response analysis is perhaps the best way to establish Proof of Concept (PoC). It is equally effective to establish causality for safety and to demonstrate proof of efficacy. It is surprising how it is seldom...
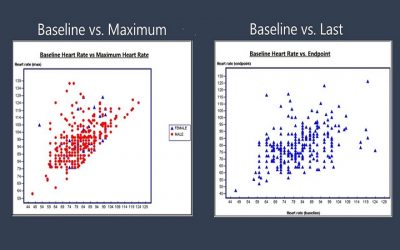
Blinded Review of Laboratory Data
Blinded review of safety laboratory data can be an onerous task. There can be close to 50 laboratory parameters distributed in just the typical hematology, chemistry and urinalysis panels. A review of tabular summaries of...
Open Label Extension Studies
A few years ago we had two situations developing in parallel. Both related to an open label extension study following a pivotal registration study. In the first instance, the Sponsor believed that it was improper to...
Milestones
Our Clientele
We have worked for U.S. and EU-based big pharma & biotech, for small molecules, biologics & cell therapy, across indications.