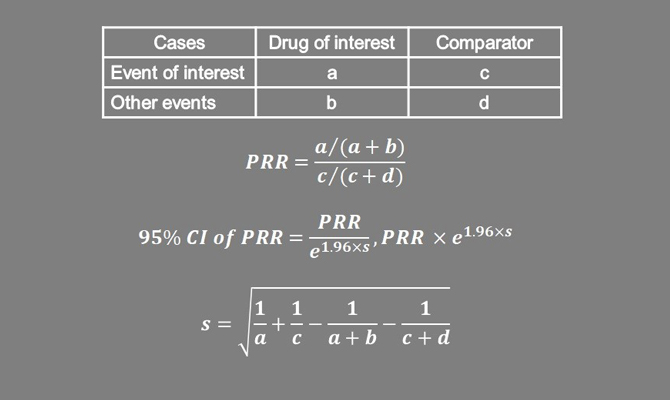
In post-marketing surveillance, proportional reporting ratio (PRR) is a useful first step for signal detection. PRR is the ratio of the proportion of adverse events of interest with the drug versus other drugs. The image above depicts the derivation of the PRR. A PRR value is considered to constitute a signal if the lower bound of the 95% Confidence Interval is ≥1 and the number of events of interest with the drug of interest is ≥3. PRR can be derived from open-source regulatory drug safety databases.
In this example, we evaluate the PRR for thromboembolic events with the Astra Zeneca COVID-19 vaccine (drug of interest) marketed in India and the UK under the brand names Covishield® and Vaxzevria® respectively. Typically, the comparator for PRR is all events for all other drugs in the database. For the purpose of this example, the comparator is other COVID-19 vaccines in use in the UK – Comirnaty® and Moderna®. We ignored vaccines for which the brand was unspecified. We used safety reports of COVID vaccines available in the public domain from the UK regulator (https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting).
The default Medical Dictionary for Regulatory Activities is MedDRA. We used the Standardised MedDRA Query (SMQ) for Embolic and thrombotic events as the event of interest.
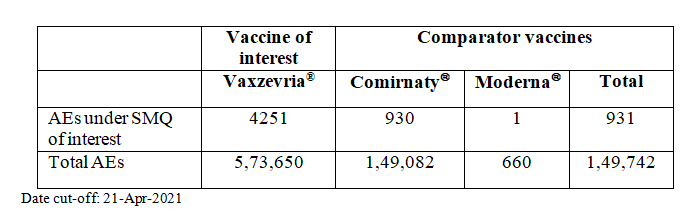
The PRR for the Embolic and thrombotic events SMQ was 1.19 (95% CI 1.11, 1.28), i.e.. there appeared to be small but statistically significant increase in thromboembolic events with Covishield® (Vaxzevria®).
A PRR is an initial step. It implies association. Once association is established, an investigation into causation commences.
Typical subsequent steps in establishing causation require a single case analysis or aggregate analysis. Overall causation is best established by using criteria like the Bradford Hill criteria used to establish the relationship between smoking and lung cancer. At times, even when association or causation is established, a drug or vaccine may not necessarily be withdrawn if the benefit risk evaluation favors ongoing use of the drug.
For more blogs, please visit: https://www.rxmd.com/insights