A few years ago we were requested by a Sponsor to undertake an analysis of outcomes of maternal exposures to a small molecule – a popular immunosuppressant. Of the different safety reports we author, these are particularly meaningful for the risk from maternal exposure is almost invariably a post-marketing risk assessment.
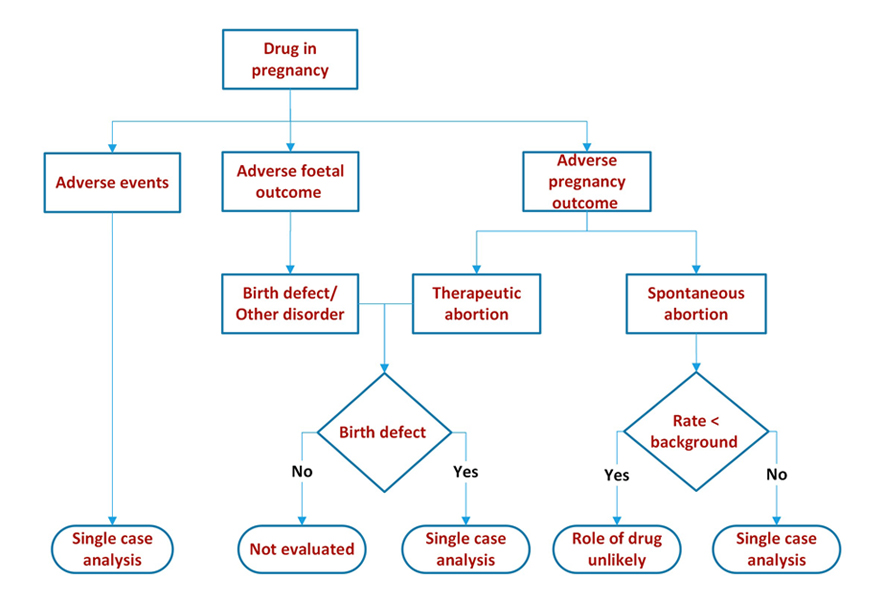
Like most ‘for cause’ safety reports, we started from scratch. Our approach was driven by the usual mantra in drug safety – for frequently occurring outcomes we compare with background rates and for infrequent yet serious events we undertake a single case analysis.
We looked at maternal, pregnancy and foetal outcomes.
Maternal outcomes – this was no different from evaluation of any post marketing adverse event.
Pregnancy outcomes – we summarized the outcomes: delivery, spontaneous abortion, still birth, therapeutic abortion, ongoing, lost to follow up or unknown by time of exposure. The time of exposure could be before conception, post conception (first, second or third trimester) or some combination of before conception and during pregnancy.
Foetal outcomes – these were summarized as normal baby, normal foetus, birth defect, other disorder (e.g. premature baby, small for gestational age, intrauterine growth retardation etc.) ongoing, lost to follow up, unknown by time of exposure as for pregnancy.
We compared event/outcome rates with background rates, proportional reporting ratios, etc. to determine if there is a signal and then proceeded with single case analysis to ascertain causality.
For more blogs, please visit: https://www.rxmd.com/insights